Custom Parts Manufacturing Solutions
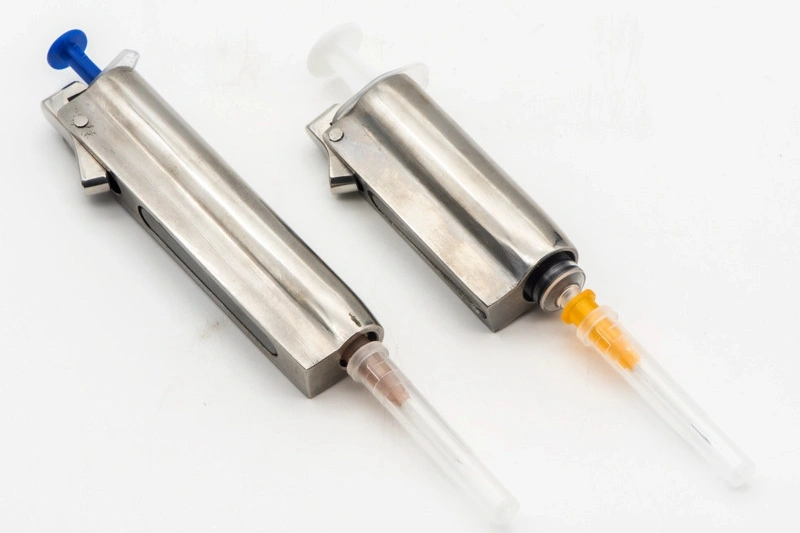
Medical Device Parts Manufacturing Service
Neway specializes in Medical Device Parts Manufacturing, offering CNC Machining, 3D Printing, Vacuum Casting, Die Casting, and Injection Molding services. We ensure high-precision, biocompatible components that meet stringent industry standards, delivering reliable and durable solutions for the medical device sector.
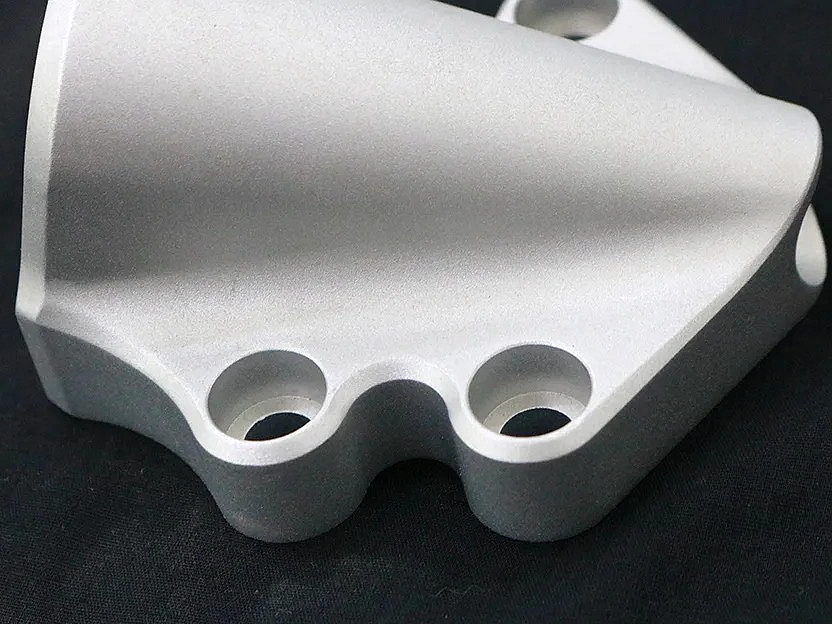
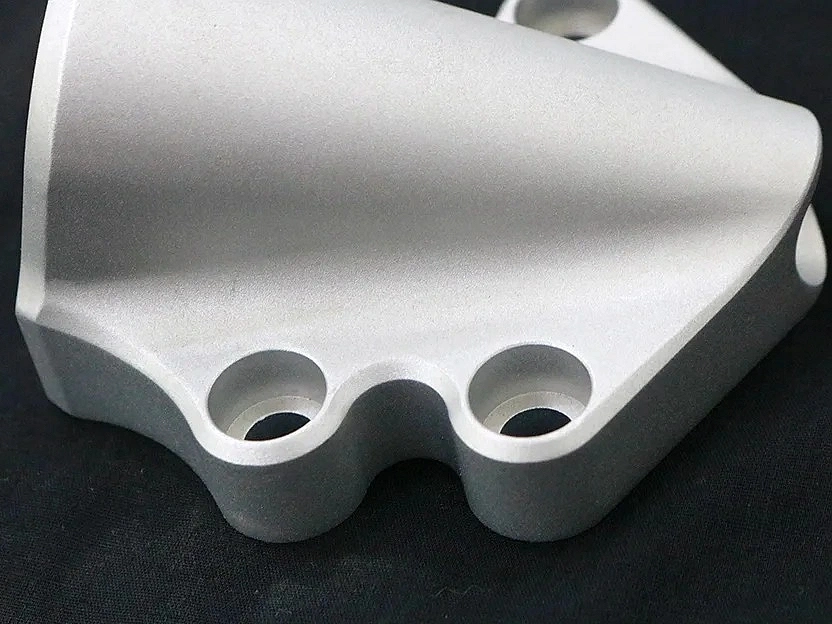
Medical Device Parts Machining
Medical device parts machining involves precise CNC processes such as milling, turning, drilling, and grinding to produce high-quality components for medical applications. Using multi-axis and precision machining techniques, along with Electrical Discharge Machining (EDM), ensures tight tolerances and reliability. These methods are essential for creating parts that meet stringent safety and performance standards in the medical industry.
Medical Device Material Selection
Materials like superalloy, titanium, aluminum, copper, brass, bronze, carbon steel, stainless steel, plastic, and ceramic are crucial in medical device manufacturing. These materials provide durability, biocompatibility, and precision for components such as implants, surgical tools, prosthetics, and medical equipment.
Typical Surface Treatment for Medical Device Parts
Typical surface treatments for medical device parts include processes like anodizing, electropolishing, PVD, powder coating, passivation, and heat treatment. These treatments enhance biocompatibility, corrosion resistance, and durability. Techniques such as electropolishing and anodizing improve surface smoothness, while coatings like Teflon or UV coatings provide additional protection, ensuring medical devices meet stringent performance and safety standards.

Learn More
Thermal Coating

Learn More
As Machined

Learn More
Painting

Learn More
PVD (Physical Vapor Deposition)
Learn More
Sandblasting
Learn More
Electroplating

Learn More
Polishing

Learn More
Anodizing

Learn More
Powder Coating

Learn More
Electropolishing

Learn More
Passivation

Learn More
Brushing

Learn More
Black Oxide

Learn More
Heat Treatment

Learn More
Thermal Barrier Coating (TBC)

Learn More
Tumbling

Learn More
Alodine

Learn More
Chrome Plating

Learn More
Phosphating

Learn More
Nitriding

Learn More
Galvanizing

Learn More
UV Coating

Learn More
Lacquer Coating

Learn More
Teflon Coating
Medical Device CNC Machining Solutions
CNC machining is crucial in medical devices, fabricating intricate components like surgical tools, implants, and diagnostic equipment, meeting strict standards for precision, biocompatibility, and safety.
Let's Start A New Project Today
Guide to Medical Device Parts Design
Medical device part design demands precision, sterility, regulatory compliance, and user safety. This guide outlines engineering principles to ensure reliable function, cleanability, and certification readiness for clinical use.
Custom Medical Device Parts Manufacturing Considerations
Custom manufacturing of medical device parts requires exceptional process control, biocompatibility, and regulatory alignment. This guide outlines critical production strategies to ensure safety, precision, and certification readiness.
Frequently Asked Questions
Explore Related Resources
Solutions
Copyright © 2026 Machining Precision Works Ltd.All Rights Reserved.